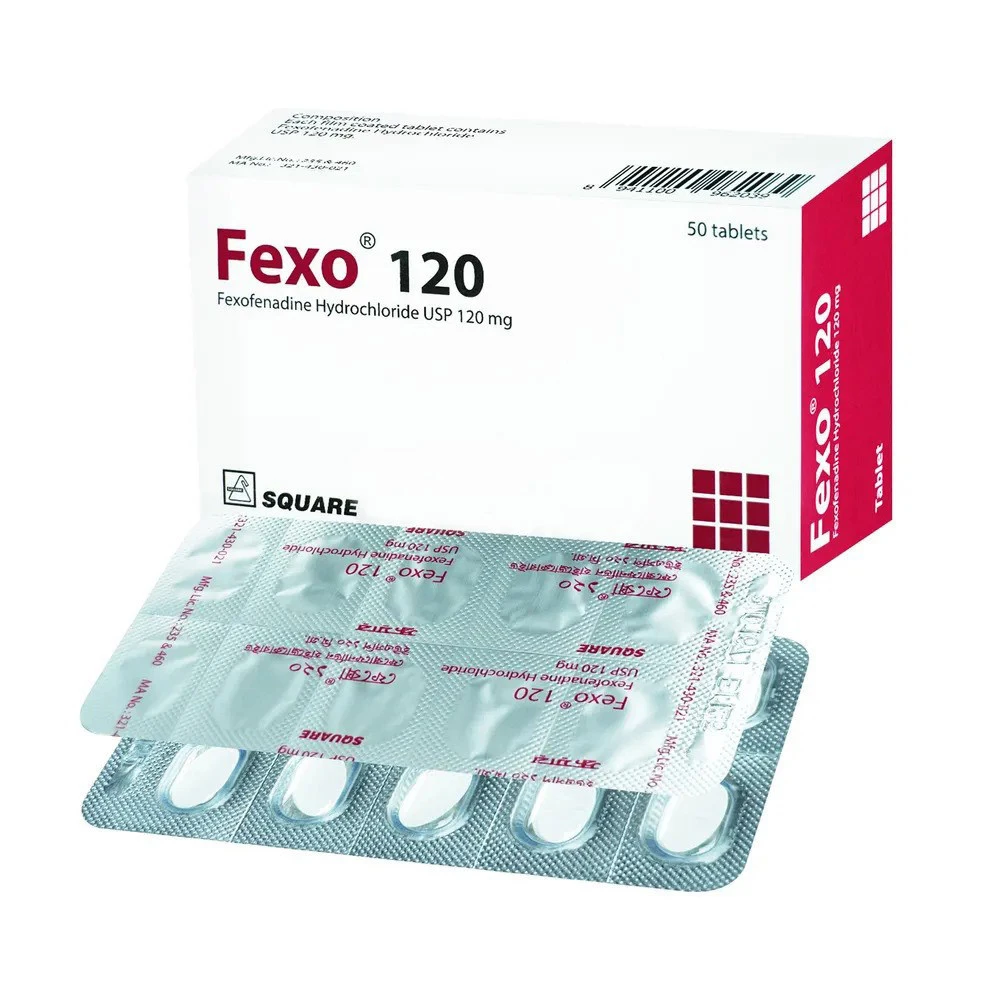
Indications of Fexo 120 mg
Fexo 120 mg is prescribed for adults and children aged 12 and older to relieve symptoms of seasonal and perennial allergic rhinitis, including sneezing, runny nose, watery or itchy eyes, and itching of the nose, throat, or palate. It is also effective for managing chronic idiopathic urticaria, reducing itching and the number of hives. Fexo 120 mg improves work productivity and quality of life for those affected by these conditions.
Therapeutic Class
Non-sedating antihistamines
Pharmacology
Fexo 120 mg, an antihistamine, selectively blocks peripheral H1 receptors. It is rapidly absorbed after oral administration, reaching peak plasma levels in 2–3 hours, and does not cross the blood-brain barrier, minimizing central nervous system effects.
Dosage & Administration of Fexo 120 mg
- Seasonal Allergic Rhinitis
- Adults and children 12+ years: 60 mg twice daily, 120 mg once daily, or 180 mg once daily (tablet). For renal impairment: 60 mg once daily.
- Children 6–11 years: 30 mg twice daily or 60 mg once daily (tablet). For renal impairment: 30 mg once daily.
- Children 2–11 years: 30 mg (5 ml) twice daily (suspension). For renal impairment: 30 mg (5 ml) once daily.
- Chronic Idiopathic Urticaria
- Adults and children 12+ years: Same as above.
- Children 6–11 years: Same as above.
- Children 6 months to <2 years: 15 mg (2.5 ml) twice daily (suspension). For renal impairment: 15 mg (2.5 ml) once daily.
- Children 2–11 years: 30 mg (5 ml) twice daily (suspension). For renal impairment: 30 mg (5 ml) once daily.
Interactions of Fexo 120 mg
Fexo 120 mg does not undergo hepatic metabolism, reducing interactions via liver pathways. Co-administration with erythromycin or ketoconazole may increase plasma levels 2–3 times, but this does not affect the QT interval or increase side effects. No interaction occurs with omeprazole. Taking Fexo 120 mg within 15 minutes of aluminum- or magnesium-containing antacids may reduce its bioavailability due to gastrointestinal binding. A 2-hour gap between Fexo 120 mg and such antacids is recommended.
Contraindications
Fexo 120 mg is contraindicated in patients with known hypersensitivity to fexofenadine or any of its ingredients.
Side Effects of Fexo 120 mg
Side effects are categorized by frequency:
- Common (≥1/100): Headache, drowsiness, dizziness, nausea.
- Uncommon (≥1/1,000): Fatigue.
- Rare/Not Known: Hypersensitivity reactions (e.g., angioedema, chest tightness, shortness of breath, flushing, anaphylaxis), insomnia, anxiety, nightmares, rapid heartbeat, palpitations, diarrhea, rash, hives, itching.
Side effects in clinical trials were similar to placebo, with additional rare effects reported post-marketing.
Pregnancy & Lactation
Fexo 120 mg is classified as Pregnancy Category C by the US FDA. It should be avoided during pregnancy and breastfeeding unless the benefits outweigh the risks to the fetus or infant. Limited data suggest fexofenadine may pass into breast milk.
Precautions & Warnings
Use cautiously in elderly patients or those with renal or hepatic impairment due to limited data. Patients with cardiovascular disease should be aware of potential antihistamine-related side effects, like rapid heartbeat or palpitations. Fexo 120 mg is unlikely to impair driving or machinery operation, but individual response should be assessed before engaging in complex tasks.
Overdose Effects of Fexo 120 mg
Overdose symptoms may include dizziness, drowsiness, fatigue, and dry mouth. Doses up to 800 mg once or 690 mg twice daily for a month showed no significant adverse effects compared to placebo. No maximum tolerated dose is established. Standard measures to remove unabsorbed drug and symptomatic treatment are recommended, as hemodialysis does not effectively clear fexofenadine.
Storage Conditions
Store in a dry place, away from light and heat, and out of reach of children.
Drug Classes
Non-sedating antihistamines
Mode of Action
Fexo 120 mg, an inverse agonist of the H1 histamine receptor, stabilizes its inactive form to prevent allergic responses. Histamine, released by mast cells and basophils during allergen exposure, triggers symptoms like itching, a runny nose, and watery eyes. Fexo 120 mg blocks these effects without crossing the blood-brain barrier, avoiding sedative or other central nervous system effects. It lacks antidopaminergic, antiserotonergic, anticholinergic, or adrenergic activity.
Pregnancy
Data on Fexo 120 mg use in pregnancy are limited, and animal studies show no adverse effects on fetal development. It should only be used if necessary. Fexofenadine may pass into breast milk, so it is not recommended for breastfeeding mothers. No human fertility studies exist, but animal studies show no impact.
Pediatric Uses
In patients with renal impairment, bioavailability and half-life of fexofenadine increase, so a reduced starting dose (e.g., 60 mg daily for adults) is advised. Hepatic impairment does not significantly alter pharmacokinetics. In patients over 65, adverse effects are similar to younger patients, but bioavailability is increased.
FAQs about Fexo 120 mg
- What is Fexo 120 mg?
Fexo 120 mg is an antihistamine that relieves allergy symptoms (e.g., hay fever, hives) by blocking histamine effects.
- Do I need a doctor’s advice before taking Fexo 120 mg?
Yes, consult your doctor to ensure Fexo 120 mg is suitable for your condition.
- Can I take Fexo 120 mg during pregnancy?
Avoid Fexo 120 mg during pregnancy unless prescribed, as it may pose risks to the fetus. Discuss safer alternatives with your doctor.
- Who should avoid Fexo 120 mg?
Avoid if allergic to fexofenadine, or if you have liver, kidney, or heart issues, are pregnant, breastfeeding, or under 12 years old.
- Is Fexo 120 mg an antibiotic?
No, Fexo 120 mg is an antihistamine, not an antibiotic, used to treat allergy symptoms.
- What are the side effects of Fexo 120 mg?
Common side effects include headache, drowsiness, dizziness, nausea, and dry mouth. Less common effects include fatigue, diarrhea, rash, or rare allergic reactions.
 Medicines
Medicines
 Healthcare
Healthcare
 Baby and Mom Care
Baby and Mom Care
 Food and Nutrition
Food and Nutrition
 Beauty
Beauty
 Home Care
Home Care
 Supplement
Supplement
 Pet Care
Pet Care
 Sexual Wellness
Sexual Wellness